Day 2 :
Keynote Forum
Yulia Desheva
Saint Petersburg State University,Russia
Keynote: Development of an associated vaccine against influenza and bacterial complications: where are we now?

Biography:
Yulia Desheva has her expertise in the preparation of vaccine strains for live influenza vaccines, the development of immunization schemes for high-risk individuals, and evaluating the role of neuraminidase antibodies in influenza infection and vaccination.
Abstract:
Statement of the Problem: Bacterial co-infections are common in influenza infected patients, including H1N1 pandemic influenza. Development of peptide vaccines based on bacterial surface proteins is a new approach to the prevention of bacterial infections. For protection against respiratory pathogens it's desirable to use mucosal vaccines, which ensures the formation of systemic immunity including secretory IgA production. This allows to reach the effective resistance against viral and bacterial pathogens. When administered intranasally, live influenza vaccine (LAIV) proved the safety and efficacy against drift variants of influenza viruses.
Methodology & Theoretical Orientation: The main objective of our research was to study the factors that contribute to the protective effect of associated immunization LAIV and Group B streptococcus (GBS) recombinant peptides. In mouse model we evaluated antibody responses against all vaccine components and followed the protection against homologous or heterologous influenza virus challenge complicated with bacterial infections.
Findings: We demonstrated that merged viral and bacterial intranasal vaccination using LAIV and recombinant GBS peptides: 1) induced balanced adaptive immune response against viral and bacterial antigens; 2) provided advantageous protection against influenza infections followed by GBS or S. pneumoniae infection compared to only LAIV-only or GBS-only vaccination; 3) the enhanced lung protection after double challenge was associated with elevated expression of IFN-gamma which may indicate T-cell immunity induction. The LAIV alone partially protected mice against S. pneumoniae secondary infection.
The Conclusion & Significance: Associated intranasal immunization using LAIV and the recombinant GBS peptides increased the protective effect against influenza and bacterial complications. Also, we conduct the study of innate immune responses against viral and bacterial antigens when administered simultaneously in monocytes-macrophages. In our study we used a short-term local application of the bacterial recombinant proteins, which further should be compared with the chimeric constructs when bacterial epitopes will be incorporated into the viral vectors.

Keynote Forum
Ronen Tchelet
Vice President of Research and Business Development , USA
Keynote: C1: How the C1 Platform Will Change the Production Approach for Recombinant Vaccines

Biography:
Ronen Tchelet joined Dyadic in May 2014 and has been Dyadic’s Vice President of Research and Business Development since January 2016. Dr. Tchelet received his Ph.D. in Molecular Microbiology and Biotechnology from Tel Aviv University in 1993 and did his postdoctoral as an EERO fellow at the Institute of Environmental Science and Technology (EAWAG) in Switzerland. In the late 2000’s, he joined the API Division of TEVA Pharmaceutical Industries LTD., where he served as a Chief Technology Officer of Biotechnology and was the QA manager of COPAXONE® the flag ship TEVA¹s innovative drug. From 2007 through 2013, prior to joining Dyadic, Dr. Tchelet became the founder and the Managing Director of Codexis Laboratories Hungary kft. (CLH) At CLH, he established a state-of-the-art laboratory for strain engineering and all aspects of fermentation work including process optimization and scaling up. It was during this time that Dr. Tchelet engaged with the C1 technology that was successfully developed for the Biofuel and the Bio-Industrial enzymes fields
Abstract:
For over 30 years Dyadic has proven itself, commercially and scientifically, as a high quality and highly productive producer of enzymes and proteins using a proprietary and patented expression system based on the Myceliopthora thermophila fungus, nicknamed C1.
The C1 platform technology is a hyper-productive fungal expression system used to develop & manufacture large quantities of desired proteins at industrial scale at significantly lower CapEx and OpEx costs.
Since the successful sale of Dyadic’s industrial biotech business to DuPont for US$75 million on December 31, 2015, we have been focused on applying the C1 technology platform to help enhance the development and manufacturing of biologic vaccines and drugs.
We achieved encouraging results, knowledge and experience in vaccine development from our prior research collaboration with Sanofi Pasteur that we believe can be leveraged and built upon with other partners. During the collaboration, meaningful improvements were made to the C1 expression system to produce antigens of interest at high level with potentially better immune response.
Dyadic is one of a consortium of companies participating in the EU sponsored ZAPI research program. ZAPI is a program sponsored by the EU suitable for the rapid development and production of vaccines and protocols to fast-track registration of developed products to combat epidemic Zoonotic diseases that have the potential to affect the human population. Insight on the development of antigens by C1 will be presented.
Dyadic has also displayed the ability to easily express mAb’s.
The C1 expression system Dyadic’s C1 technology has the potential to change the way in which both animal health and human biotech and pharmaceutical companies bring their biologic vaccines and drugs to market faster, in greater volumes, at lower cost, and with newer beneficial properties, and most importantly save lives.
Dyadic believes that our current efforts, with or without potential partners, to successfully express several therapeutic proteins, will validate the C1 technology as one of the vital production platforms for developing & manufacturing biologic Vaccines and BioPharmaceuticals
Keynote Forum
Jwee Chiek
veterinary epidemiologist and a research scientist at the Norwegian Veterinary Institute, NORWAY
Keynote: Occurrence and spread of influenza A(H1N1)pdm09 virus infection in Norwegian pig herds based on active serosurveillance from 2010 to 2014

Biography:
Jwee Chiek Er is a veterinary epidemiologist and a research scientist at the Norwegian Veterinary Institute. After graduating with BVSc at the University of Queensland, Australia in 1989, he worked as a public health inspector at slaughterhouses for pigs and poultry in Singapore for 13 years. He obtained a Master in Preventative Veterinary Medicine (MPVM) at UC Davis in 2000 and began his career as an epidemiologist. With the pandemic H1N1 virus emerging in the world and infecting the pigs in Norway for the first time for any influenza strain, he spent 5 five years conducting epidemiological research of this new influenza strain in Norwegian pigs and investigated production impact it has on pig production. His publications are listed as above. He received his Doctorate in Philosophy for his research in 2016 at the University of Life Sciences in Oslo.
Abstract:
The incursion of influenza A(H1N1)pdm09 virus was detected by Norway’s active serosurveillance of its pig population in 2009. Since then, surveillance data from 2010 to 2014 revealed that 54% of 5643 herd tests involving 1567 pig herds and 28% of 23 036 blood samples screened positive for antibodies against influenza A virus. Positive herds were confirmed to have influenza A(H1N1)pdm09 virus infection by haemagglutination inhibition test. In 50% of positive herd tests, 560% of the sampled pigs in each herd had antibodies against influenza A(H1N1) pdm09 virus. This within-herd animal seroprevalence did not vary for type of production, herd size or year of test. The overall running mean of national herd seroprevalence, and annual herd incidence risks fluctuated narrowly around the means of 45% and 32%, respectively, with the highest levels recorded in the three densest pig-producing counties. The probability of a herd being seropositive varied in the five production classes, which were sow pools, multiplier herds, conventional sow herds, nucleus herds, and fattening herds in descending order of likelihood. Large herds were more likely to be seropositive. Seropositive herds were highly likely to be seropositive the following year. The study shows that influenza A(H1N1)pdm09 virus is established in the Norwegian pig population with recurrent and new herd infections every year with the national herd seroprevalence in 2014 hovering at around 43% (95% confidence interval 40–46%).
Keynote Forum
Hejer Harrabi
ElManar University of Tunis,TUNISIA
Keynote: Knowledge, attitudes and beliefs related to seasonal Influenza vaccination among Tunisian physicians

Biography:
Hejer Harrabi, is an university medical doctor, infectious diseases specialist, La Rabta University Hospital, Tunis El Manar University,Tunisia. Experience in France: clinical research assistant, referring doctor. Studies: University François Rabelais, Sfax Faculty of Medicine. Interests of study: Influenza, HIV nfection, viral hepatitis, travel medicine, tuberculosis, bone and joint infections
Abstract:
Statement of the problem: Despite the recommendations for physicians, nurses, and other personnel in both hospital and outpatient-care settings to be vaccinated annually against influenza, the influenza vaccination rate among Tunisian healthcare workers (HCW) remains low.
The purpose of this survey is to assess Influenza vaccination status and related knowledge, attitudes and beliefs among a national sample of primary care physicians and specialists likely to see patients at high risk for complications from Influenza.
Methodology: We are conducting a prospective cross-sectional survey in Tunis (Tunisia) from February 2017 to April 2017. A self-administered questionnaire covering knowledge, attitudes and beliefs related to Influenza was mailed to a sample of physicians who likely to see patients at high risk for complications from Influenza. Herein, we present the first results of the study during February 2017.
Results: In a first mailing, during February 2017, 150 physicians were included. The overall response was 54.6% (n=82). Physicians reported a very low vaccination rate: 12% (n=10). Of the 72 unvaccinated, 33.3 % considered low risk of catching or spreading Influenza, 26.3% did not have access to vaccine on site, 16.6 % feared the side effects of the vaccine and 5.5% considered influenza to be a benign illness. All the physicians recommend vaccination for their patients at high risk for complications from Influenza. They were also asked about the HCWs vaccination status in their departments: 26.8% of the physicians estimated that HCWs were vaccinated.
Keynote Forum
Asma AYARI
François TROTTEIN,FRANCE
Keynote: Adipose tissue: a neglected reservoir for influenza virus?

Biography:
Asma AYARI is a PhD student, she is starting her first steps in research after a Master degree in Microbiology, which allowed her to acquire knowledge in the field of bacterial screening. Interested about Medical and health science, for her thesis project she works on understanding the association between obesity and pulmonary infections working on a viral model (Influenza Virus) and a bacterial model (Streptococcus pneumonia). This experience allowed her to make the link between the Immune aspect of the infection and the potential metabolic impacts.
Abstract:
Statement of the problem: Influenza type A virus (IAV), represents a worldwide threat to human health. Recently, it has been reported that besides young and old subjects, obese patients also present an increased susceptibility to IAV infection. Since obesity is associated with an excessive white adipose tissue (WAT) expansion, we questioned whether IAV could impact WAT and its main cellular components.
Methodology & Theoretical Orientation: C57/BL6 lean and high-fat diet-induced obese mice were infected with IAV (virus strain: H3N2, dose: 30 PFUs/mouse, route: intra-nasal). At 7 days post-infection, lungs, WAT, pancreas and liver were harvested for analysis of viral (M1 protein) and antiviral proteins gene expression. To analyze the impact of IAV infection on adipose tissue, WAT explants secretion from infected or uninfected lean and obese mice were analyzed. Then, to see whether the virus could target preadipocytes and mature adipocytes, we infected differentiated, or not, 3T3-L1 cell line. Cells were harvested for transcriptional analyses (tested genes: M1, Tlr3 and Mda5) and Supernatants were collected for the detection of newly formed virions and cytokines.
Findings: In vivo data showed that viral genome could be detected in the adipose tissue of infected mice. Moreover, in response to the infection, the adipose tissue produce more pro- and anti-inflammatory cytokines, thus demonstrating that IAV infection does impact WAT inflammatory state. In vitro tests showed that preadipocytes can be infected by IAV, yet without virion release.
Keynote Forum
Arvind Singh Kushwaha
Station Health Organisation, INDIA
Keynote: Pandemic influenza: Experience in a flu OPD of a tertiary care hospital

Biography:
Dr Arvind Singh Kushwaha graduated from Armed Forces Medical College in 1989 and did his MD in 1998 in Preventive and Social Medicine from Pune University. He is presently health officer of a Cantonment and a UG and PG teacher in the AFMC Pune. He has published more than 20 papers in reputed journals and has been awarded twice for best Research article in Community Medicine. His interest include infectious disease, outbreak investigation, social medicine and mental health amongst soldiers.
Abstract:
Background: In April 2009, Mexican health authorities announced an outbreak of a novel H1N1 influenza virus, which subsequently caused a pandemic. The world is now moving into the post-pandemic period. The experience gained in handling this pandemic at various levels under different settings has provided us many lessons for the future.
Objective: To study the profile of various activities undertaken at flu screening centre as a response to pandemic influenza in a tertiary care hospital.
Methods: Record-based study conducted in a tertiary care hospital of Pune. Required data was collected from records of flu OPD, ward and local health authority and interviewing related staff. Study included data from October 2009 to October 2010.
Results: A total of 8020 people presenting with influenza like illness (ILI) were screened in the flu OPD under study. Out of these, only 388 (4.84%) met clinical criteria where throat samples were collected, out of which only 81 were found to be positive (20.88%). Total three fatalities (3.7%) occurred out of 81 who had tested positive. Most cases of flu were managed at home (76.54%) while only 19 (23.4%) lab confirmed cases of H1N1 required hospitalisation.
Conclusion: Majority of cases of H1N1 (2009) were managed at home. Early diagnosis, quick initiation of treatment, infection control measures, and good care at the hospital can effectively reduce morbidity and mortality in H1N1 pandemic.
Keynote Forum
Ronen Tchelet
Dyadic Inc, USA
Keynote: C1: How the C1 Platform Will Change the Production Approach for Recombinant Vaccines

Biography:
Ronen Tchelet joined Dyadic in May 2014 and has been Dyadic’s Vice President of Research and Business Development since January 2016. Dr. Tchelet received his Ph.D. in Molecular Microbiology and Biotechnology from Tel Aviv University in 1993 and did his postdoctoral as an EERO fellow at the Institute of Environmental Science and Technology (EAWAG) in Switzerland. In the late 2000’s, he joined the API Division of TEVA Pharmaceutical Industries LTD., where he served as a Chief Technology Officer of Biotechnology and was the QA manager of COPAXONE® the flag ship TEVA¹s innovative drug. From 2007 through 2013, prior to joining Dyadic, Dr. Tchelet became the founder and the Managing Director of Codexis Laboratories Hungary kft. (CLH) At CLH, he established a state-of-the-art laboratory for strain engineering and all aspects of fermentation work including process optimization and scaling up. It was during this time that Dr. Tchelet engaged with the C1 technology that was successfully developed for the Biofuel and the Bio-Industrial enzymes fields.
Abstract:
For over 30 years Dyadic has proven itself, commercially and scientifically, as a high quality and highly productive producer of enzymes and proteins using a proprietary and patented expression system based on the Myceliopthora thermophila fungus, nicknamed C1.
The C1 platform technology is a hyper-productive fungal expression system used to develop & manufacture large quantities of desired proteins at industrial scale at significantly lower CapEx and OpEx costs.
Since the successful sale of Dyadic’s industrial biotech business to DuPont for US$75 million on December 31, 2015, we have been focused on applying the C1 technology platform to help enhance the development and manufacturing of biologic vaccines and drugs.
We achieved encouraging results, knowledge and experience in vaccine development from our prior research collaboration with Sanofi Pasteur that we believe can be leveraged and built upon with other partners. During the collaboration, meaningful improvements were made to the C1 expression system to produce antigens of interest at high level with potentially better immune response.
Dyadic is one of a consortium of companies participating in the EU sponsored ZAPI research program. ZAPI is a program sponsored by the EU suitable for the rapid development and production of vaccines and protocols to fast-track registration of developed products to combat epidemic Zoonotic diseases that have the potential to affect the human population. Insight on the development of antigens by C1 will be presented.
Dyadic has also displayed the ability to easily express mAb’s.
The C1 expression system Dyadic’s C1 technology has the potential to change the way in which both animal health and human biotech and pharmaceutical companies bring their biologic vaccines and drugs to market faster, in greater volumes, at lower cost, and with newer beneficial properties, and most importantly save lives.
Dyadic believes that our current efforts, with or without potential partners, to successfully express several therapeutic proteins, will validate the C1 technology as one of the vital production platforms for developing & manufacturing biologic Vaccines and BioPharmaceuticals.
- Track 1:Pathogenicity of Influenza Virus
Track 3:Influenza: Causes, Symptoms and Treatment
Track 5:Zoonotic Diseases: Global Infectious Disease Burden
Location: Birmingham, UK
Session Introduction
Sherwin Morgan
University of Chicago Medicine
USA
Title: Recognition of Influenza and non-Influenza Related Respiratory Illness

Biography:
Sherwin Morgan completed his respiratory care training from Malcolm X College of Respiratory Care in Chicago, IL. He is an advanced respiratory care practitioner with the National Board for Respiratory Care in the United States. He is Clinical Practice and Development /Educator/Research Coordinator for the Department of Respiratory Care Services, Section of Pulmonary and Critical Care Medicine at the University of Chicago Medicine. He has published more than 25 peer review papers in multiple medical journals. He has designed, engineered, and collaborated with a number of research studies with the pulmonary medicine department.
Abstract:
Recognition of respiratory illness (RI) is difficult and requires a respiratory viral panel (RVP) to assist with establishing an accurate clinical diagnosis. Influenza and non-influenza like respiratory illness often masquerades as asthma-like. Because the initial differential diagnosis includes asthma, this can lead to treatment confusion and an underestimation for the primary causes of air-flow obstruction. Viral related bronchospasm with air-flow obstruction (AFO) is difficult to ameliorate with bronchodilator therapy when associated with bronchiolitis. Emerging research from histopathology of rat lung tissue study is providing valuable information as to how many viral agents affect lung pathophysiology. These viruses are high pathogenic and may cause a change in bronchial wall structure, peri bronchial thickening and intravascular hemorrhage. These viruses may be the root cause of global epidemics and pandemics. Globally the H1N1 pandemic 2009 caused over 18,000 deaths. Non-influenza viruses such as enterovirus D-68 has been having a profound effect globally and responsible for deaths in the USA and Philippines. Zoonotic viruses such as Coronavirus 229E has been linked to Middle East Severe Acute Respiratory Syndrome (MERS). Many viruses are passed bi-directionally between animal and humans, 2017 the USA had a dog flu epidemic. Viral lung infections are known to increase morbidity and mortality in patients with and without premorbid pulmonary disease. They are highly pathogenic and are known to increase mortality in patients with compromised immunes systems. Failure to recognize acute RI may respiratory failure where the support therapy is ventilator, proning, nitric oxide, ECMO. This can lead to complications such as; ARDS, and organ failure and severe acute respiratory syndrome. There have been case reports which indicated that high flow nasal cannula and heliox may be effective as supportive therapy. More study is needed to understand the relationship between acute RI and these support therapies.

Biography:
Vincent Icheku BSc (Hons), M.Phil., PhD is a senior lecturer in the School of health and social care, London South Bank University, United Kingdom.
I received senior fellowship award in 2014; a national award for my contribution to UK higher education and reaching worldwide audience with my work by the UK Higher Education Academy. I am also, a nominee for the London South Bank University Best Teacher’s Award for 2015 and 2016 respectively.
My subject teaching expertise includes research methods, Public health/Health promotion, Social policies, Concept of interprofessional working in practice, Ethics and law. Research interest is in community and public health, published books and many Journal articles. He is currently Editor of UK Research Journal and a Senior Fellow, Higher Education Academy (SFHEA) and Fellow, Royal Society of Public Health (FRSPH).
Abstract:
Statement of the problem:
The World Health Organization (WHO) in May 2016 confirmed an outbreak of the Zika virus on the African island chain of Cape Verde, linking it to cases of the brain disease, microcephaly. This finding is of concern because Zika was first discovered in East Africa in 1947 with no known link to brain or birth disorders until the WHO reported findings. The question, therefore, is: if the Zika virus has been in Africa for 70 years, why wasn’t any association to microcephaly detected before the recent WHO findings in Brazil (see below) and Cape Verde? This study reviews the evidence linking Zika to microcephaly in view of recent cases of birth defects in Africa, with the aim of providing vital clues as to why there was no documented case of such birth defects in Africa, where the Zika virus originated.
Review methodology:
The literature for this review was gathered through internet searches, including the websites of the European Centre for Disease Prevention and Control (ECDC), the United States Centre for Disease Control and Prevention (CDC), the World Health Organization (WHO) and Public Health England (PHE).
Findings:
Materials from these sources were reviewed on the link between the Zika virus and microcephaly in relation to the recent cases of birth defects in Africa. Two possible explanations emerged from the review. The first explanation suggests that the phenomenon called herd immunity may have taken place in Africa. The Zika virus cannot infect the same person twice because it reaches a stage where there are too few people left to be infected for transmission to be sustained. The second explanation suggests that microcephaly linked to birth defects is caused by other conditions.
In conclusion:
The findings of this review opens up the debate on the connection between the Zika virus and the birth defect attributed to mosquito-borne microcephaly, given that there is no documented case of birth defect in Africa 69 years after the discovery of the Zika virus. Large-scale research is recommended on the Zika virus and pregnancy in Africa for better understanding of the ecology and epidemiology of the virus in the continent.
Edmond Puca
University Hospital Center
ALBANIA
Title: Some dates about leptospirosis in Albania and the role of gender predisposition in leptospirosis

Biography:
Edmond Puca MD, PhD. He is an infectious diseases specialist. His fields of interest are tropical infectious diseases, especially leptospirosis and hemorrhagic fever with renal syndrome. He has published a lot of paper in this field. He is member of editorial board of some infectious diseases journals and the scientific secretary congress committee
Abstract:
Leptospirosis is a zoonotic spirochetal disease of global importance especially in developing countries, which continues to have a major impact on public health. The incidence of the disease is much higher in males.
Objectives: To describe the evidence regarding to the role of gender in leptospirosis, through a gender specific analysis of the clinical manifestations in patients diagnosed and treated for leptospirosis in our infectious diseases service during the 2006-2015 period and to give some data about leptospirosis in Albania for the last ten years.
Materials and methodology: We reviewed the epidemiologic data, risk factors and differences in clinical presentation between male and female patients. Diagnosis was established based on clinical presentation subsequently confirmed serologically by Anti-Leptospira IgM antbibodies through ELISA test.
Results: Between 2006-2015, 206 cases of confirmed leptospirosis were analysed. 185 (89.8%) were males and 21 patients (10.2%) were females. The highest incidence was observed in the 45-64 age groups. Overall mortality was found to be 7.7%. Mortality among female patients was 4.7%, whereas among males was 8.1%. Chi-square statistical analysis showed a p value of 0.577045, demonstrating no statistical significance in mortality between two genders.
Conclusions: There is a much higher incidence of leptospirosis in middle aged men. Mortality rate seems to be similar in males and female. While the difference in incidence may be related to exposure to risk factors, we believe that further investigations are necessary to study gender-based genetic and immunological predisposition.
Misgana Bancha1
Ethiopian Public Health Institute
Title: Influenza Outbreak Investigation, Dangur District, Ethiopia, 2016

Biography:
Misgana has completed her Bsc on Public Health from Hawassa University and Since joining Ethiopian Public Health Institute in 2013, she has been working in Public Health Emergency Management department as Early Warning and Response Team officer and has been involved with studies related to public health emergencies and then become assistant researcher. Currently, she is studying Masters of Public Health in Field Epidemiology at St. Paul’s Hospital
Abstract:
Influenza is a significant source of morbidity and mortality and is estimated to result in up to five million cases and 250,000 to 500,000 deaths worldwide each year. The outbreak reported from Dangur district on 18-March-2016. We investigated to identify risk factors and to recommend control and prevention measures. Unmatched case control study was employed. Study subjects (50 cases, 100 controls) were interviewed. Cases were defined as any person, with sudden onset of fever >38ºC and cough or sore throat in the absence of other diagnosis. Controls were any person having the same characteristics with cases except having history of the above signs and symptoms. Medical records reviewed and suspected cases were identified from 09-Feb-2016 to 07-Apr-2016. Nine throat swab samples taken to confirm the diagnosis. Data was analyzed by using Epi-info version 7.1. We identified 433-suspected influenza cases with three deaths. Of the cases, 235(54.3%) were males. Attack-Rate was 6.8 per 1000 (7.3 in males and 6.2 in females per 1000). It affected all age groups with highest AR (18 per 1000) among 0-4 age groups. Factors associated with illness were having close contact with patient (OR: 3.27; 95%CI: 1.4-7.4, p=0.0044) and age (OR: 0.09; 95%CI: 0.03-0.2, p=0.00). From the samples tested, seven confirmed positive. The study uncovered the occurrence of pdmH1N1 influenza virus in Dangur district. Having contact with cases is 4.1 times more likely to contract the disease and being at age group >5years is 0.09 times protective of contracting the disease. Strengthening routine surveillance is recommended.
Ketema Misganaw
Ethiopian Public Health Institute
ETHIOPIA
Title: Investigation of Influenza Like Illness Outbreak-Argoba Special District, South Wollo, Amhara Region, Ethiopia, April, 2016

Biography:
Ketema has completed her BSC degree in public health at the age of 21 years from University of Gondar and post graduate studies at Saint Paul's Hospital Millennium Medical colleague Department of Public Health. She is Assistant Researcher I in Public Health Emergency Management center of Ethiopian Public Health Institute, Addis Ababa, Ethiopia. She has done Surveillance data analysis, Health profile, Outbreak investigation and Surveillance system evaluation .
Abstract:
Influenza is an acute viral respiratory tract disease characterized by fever, headache, myalgia, prostration, coryza, sore throat and cough. We investigated investigated to identify the risk factors and to control the transmission and finally to come up with prevention and control measures. Unmatched case control study design was employed. Exposure and risk factor information was collected by face to face interview of cases and controls by using structured questionnaire. A total of 50 cases and 100 controls were taken . Median age is 26 year old for both cases and controls. On multivariate analysis only having contact history with similar patient(s) was statistically significantly associated with illness (OR: 19.5; 95%CI: 5.99-63.67; P value<0.001). Of the total 11 samples collected and tested by RT-PCR only seven were positive for influenza type A and sub types (six were pdmH1N1 and one H3N2 ) and four were negative result. Therefore, the cause of outbreak was influenza type A pdm H1N1. This investigation found that the outbreak primarily affected young children and young adults. clinical pictures. Since the causative agent and mode of transmissions responsible for the outbreak were known the prevention and control measures should be under taken and strengthen.
- Track 2:Influenza Vaccines and Vaccinnation
Track 7:Tracking and Preventing Zoonotic Disease
Track 12:Neglected Tropical and Communicable diseases
Track 13:Evolution and Epidemiological Aspects of Influenza and Zoonotic Diseases
Location: Birmingham, UK
Session Introduction
Ronen Tchelet
Dyadic Inc, USA
Title: C1: How the C1 Platform Will Change the Production Approach for Recombinant Vaccines

Biography:
Ronen Tchelet joined Dyadic in May 2014 and has been Dyadic’s Vice President of Research and Business Development since January 2016. Dr. Tchelet received his Ph.D. in Molecular Microbiology and Biotechnology from Tel Aviv University in 1993 and did his postdoctoral as an EERO fellow at the Institute of Environmental Science and Technology (EAWAG) in Switzerland. In the late 2000’s, he joined the API Division of TEVA Pharmaceutical Industries LTD., where he served as a Chief Technology Officer of Biotechnology and was the QA manager of COPAXONE® the flag ship TEVA¹s innovative drug. From 2007 through 2013, prior to joining Dyadic, Dr. Tchelet became the founder and the Managing Director of Codexis Laboratories Hungary kft. (CLH) At CLH, he established a state-of-the-art laboratory for strain engineering and all aspects of fermentation work including process optimization and scaling up. It was during this time that Dr. Tchelet engaged with the C1 technology that was successfully developed for the Biofuel and the Bio-Industrial enzymes fields.
Abstract:
For over 30 years Dyadic has proven itself, commercially and scientifically, as a high quality and highly productive producer of enzymes and proteins using a proprietary and patented expression system based on the Myceliopthora thermophila fungus, nicknamed C1.
The C1 platform technology is a hyper-productive fungal expression system used to develop & manufacture large quantities of desired proteins at industrial scale at significantly lower CapEx and OpEx costs.
Since the successful sale of Dyadic’s industrial biotech business to DuPont for US$75 million on December 31, 2015, we have been focused on applying the C1 technology platform to help enhance the development and manufacturing of biologic vaccines and drugs.
We achieved encouraging results, knowledge and experience in vaccine development from our prior research collaboration with Sanofi Pasteur that we believe can be leveraged and built upon with other partners. During the collaboration, meaningful improvements were made to the C1 expression system to produce antigens of interest at high level with potentially better immune response.
Dyadic is one of a consortium of companies participating in the EU sponsored ZAPI research program. ZAPI is a program sponsored by the EU suitable for the rapid development and production of vaccines and protocols to fast-track registration of developed products to combat epidemic Zoonotic diseases that have the potential to affect the human population. Insight on the development of antigens by C1 will be presented.
Dyadic has also displayed the ability to easily express mAb’s.
The C1 expression system Dyadic’s C1 technology has the potential to change the way in which both animal health and human biotech and pharmaceutical companies bring their biologic vaccines and drugs to market faster, in greater volumes, at lower cost, and with newer beneficial properties, and most importantly save lives.
Dyadic believes that our current efforts, with or without potential partners, to successfully express several therapeutic proteins, will validate the C1 technology as one of the vital production platforms for developing & manufacturing biologic Vaccines and BioPharmaceuticals.
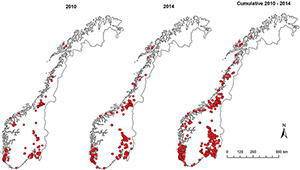
Jwee Chiek
Norwegian Veterinary Institute, NORWAY
Title: Occurrence and spread of influenza A(H1N1)pdm09 virus infection in Norwegian pig herds based on active serosurveillance from 2010 to 2014

Biography:
Jwee Chiek Er is a veterinary epidemiologist and a research scientist at the Norwegian Veterinary Institute. After graduating with BVSc at the University of Queensland, Australia in 1989, he worked as a public health inspector at slaughterhouses for pigs and poultry in Singapore for 13 years. He obtained a Master in Preventative Veterinary Medicine (MPVM) at UC Davis in 2000 and began his career as an epidemiologist. With the pandemic H1N1 virus emerging in the world and infecting the pigs in Norway for the first time for any influenza strain, he spent 5 five years conducting epidemiological research of this new influenza strain in Norwegian pigs and investigated production impact it has on pig production. His publications are listed as above. He received his Doctorate in Philosophy for his research in 2016 at the University of Life Sciences in Oslo
Abstract:
The incursion of influenza A(H1N1)pdm09 virus was detected by Norway’s active serosurveillance of its pig population in 2009. Since then, surveillance data from 2010 to 2014 revealed that 54% of 5643 herd tests involving 1567 pig herds and 28% of 23 036 blood samples screened positive for antibodies against influenza A virus. Positive herds were confirmed to have influenza A(H1N1)pdm09 virus infection by haemagglutination inhibition test. In 50% of positive herd tests, 560% of the sampled pigs in each herd had antibodies against influenza A(H1N1) pdm09 virus. This within-herd animal seroprevalence did not vary for type of production, herd size or year of test. The overall running mean of national herd seroprevalence, and annual herd incidence risks fluctuated narrowly around the means of 45% and 32%, respectively, with the highest levels recorded in the three densest pig-producing counties. The probability of a herd being seropositive varied in the five production classes, which were sow pools, multiplier herds, conventional sow herds, nucleus herds, and fattening herds in descending order of likelihood. Large herds were more likely to be seropositive. Seropositive herds were highly likely to be seropositive the following year. The study shows that influenza A(H1N1)pdm09 virus is established in the Norwegian pig population with recurrent and new herd infections every year with the national herd seroprevalence in 2014 hovering at around 43% (95% confidence interval 40–46%).
Hejer Harrabi
ElManar University of Tunis, Tunisia
Title: Knowledge, attitudes and beliefs related to seasonal Influenza vaccination among Tunisian physicians

Biography:
Hejer Harrabi, is an university medical doctor, infectious diseases specialist, La Rabta University Hospital, Tunis El Manar University,Tunisia.Experience in France: clinical research assistant, referring doctor. Studies: University François Rabelais, Sfax Faculty of Medicine. Interests of study: Influenza, HIV nfection, viral hepatitis, travel medicine, tuberculosis, bone and joint infections
Abstract:
Statement of the problem: Despite the recommendations for physicians, nurses, and other personnel in both hospital and outpatient-care settings to be vaccinated annually against influenza, the influenza vaccination rate among Tunisian healthcare workers (HCW) remains low.
The purpose of this survey is to assess Influenza vaccination status and related knowledge, attitudes and beliefs among a national sample of primary care physicians and specialists likely to see patients at high risk for complications from Influenza.
Methodology: We are conducting a prospective cross-sectional survey in Tunis (Tunisia) from February 2017 to April 2017. A self-administered questionnaire covering knowledge, attitudes and beliefs related to Influenza was mailed to a sample of physicians who likely to see patients at high risk for complications from Influenza. Herein, we present the first results of the study during February 2017.
Results: In a first mailing, during February 2017, 150 physicians were included. The overall response was 54.6% (n=82). Physicians reported a very low vaccination rate: 12% (n=10). Of the 72 unvaccinated, 33.3 % considered low risk of catching or spreading Influenza, 26.3% did not have access to vaccine on site, 16.6 % feared the side effects of the vaccine and 5.5% considered influenza to be a benign illness. All the physicians recommend vaccination for their patients at high risk for complications from Influenza. They were also asked about the HCWs vaccination status in their departments: 26.8% of the physicians estimated that HCWs were vaccinated.
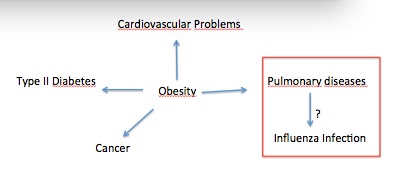
Asma AYARI
François TROTTEIN Isabelle WOLOWCZUK, fRANCE
Title: Adipose tissue: a neglected reservoir for influenza virus?

Biography:
Asma AYARI is a PhD student, she is starting her first steps in research after a Master degree in Microbiology, which allowed her to acquire knowledge in the field of bacterial screening. Interested about Medical and health science, for her thesis project she works on understanding the association between obesity and pulmonary infections working on a viral model (Influenza Virus) and a bacterial model (Streptococcus pneumonia). This experience allowed her to make the link between the Immune aspect of the infection and the potential metabolic impacts.
Abstract:
Statement of the problem: Influenza type A virus (IAV), represents a worldwide threat to human health. Recently, it has been reported that besides young and old subjects, obese patients also present an increased susceptibility to IAV infection. Since obesity is associated with an excessive white adipose tissue (WAT) expansion, we questioned whether IAV could impact WAT and its main cellular components.
Methodology & Theoretical Orientation: C57/BL6 lean and high-fat diet-induced obese mice were infected with IAV (virus strain: H3N2, dose: 30 PFUs/mouse, route: intra-nasal). At 7 days post-infection, lungs, WAT, pancreas and liver were harvested for analysis of viral (M1 protein) and antiviral proteins gene expression. To analyze the impact of IAV infection on adipose tissue, WAT explants secretion from infected or uninfected lean and obese mice were analyzed. Then, to see whether the virus could target preadipocytes and mature adipocytes, we infected differentiated, or not, 3T3-L1 cell line. Cells were harvested for transcriptional analyses (tested genes: M1, Tlr3 and Mda5) and Supernatants were collected for the detection of newly formed virions and cytokines.
Findings: In vivo data showed that viral genome could be detected in the adipose tissue of infected mice. Moreover, in response to the infection, the adipose tissue produce more pro- and anti-inflammatory cytokines, thus demonstrating that IAV infection does impact WAT inflammatory state. In vitro tests showed that preadipocytes can be infected by IAV, yet without virion release.
Arvind Singh Kushwaha
Station Health Organisation, INDIA
Title: Title: Pandemic influenza: Experience in a flu OPD of a tertiary care hospital

Biography:
Dr Arvind Singh Kushwaha graduated from Armed Forces Medical College in 1989 and did his MD in 1998 in Preventive and Social Medicine from Pune University. He is presently health officer of a Cantonment and a UG and PG teacher in the AFMC Pune. He has published more than 20 papers in reputed journals and has been awarded twice for best Research article in Community Medicine. His interest include infectious disease, outbreak investigation, social medicine and mental health amongst soldiers.
Abstract:
Background: In April 2009, Mexican health authorities announced an outbreak of a novel H1N1 influenza virus, which subsequently caused a pandemic. The world is now moving into the post-pandemic period. The experience gained in handling this pandemic at various levels under different settings has provided us many lessons for the future.
Objective: To study the profile of various activities undertaken at flu screening centre as a response to pandemic influenza in a tertiary care hospital.
Methods: Record-based study conducted in a tertiary care hospital of Pune. Required data was collected from records of flu OPD, ward and local health authority and interviewing related staff. Study included data from October 2009 to October 2010.
Results: A total of 8020 people presenting with influenza like illness (ILI) were screened in the flu OPD under study. Out of these, only 388 (4.84%) met clinical criteria where throat samples were collected, out of which only 81 were found to be positive (20.88%). Total three fatalities (3.7%) occurred out of 81 who had tested positive. Most cases of flu were managed at home (76.54%) while only 19 (23.4%) lab confirmed cases of H1N1 required hospitalisation.
Conclusion: Majority of cases of H1N1 (2009) were managed at home. Early diagnosis, quick initiation of treatment, infection control measures, and good care at the hospital can effectively reduce morbidity and mortality in H1N1 pandemic.
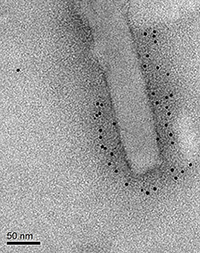
Yu-Chan Chao
Latter Institute,Taiwan
Title: Anchorage of Influenza Virus Hemagglutinin onto Baculovirus as a Convenient Tool for Antibody Production

Biography:
Yu-Chan Chao received a Ph. D. from University of Arkansas, and completed his postdoctoral training at the Cold Spring Harbor Laboratory and Cornell University. He previously served as the Dean of the College of Life Science, National Chung-Hsing University and as Deputy Director at the Institute of Molecular Biology, Academia Sinica, and is now a professor at this latter institute. He has received three Distinguished Research Awards from the Ministry of Science and Technology, Taiwan, and was elected as a Distinguished Research Fellow. He is a council member of the International Congress of Entomology, and has published more than 70 papers thus far. He also serves as an editor for several highly-regarded international journals
Abstract:
Influenza virus is an important cause of diseases circulating between humans and animals. On the envelope of this virus, Hemagglutinin (HA) is the major antigen that is recognizable by the animal immune system upon viral infection. Due to the potential of viral infection, it is difficult to acquire or manipulate influenza virus. It is also difficult to purify HA (a membrane protein) as a vaccine. In order to resolve these problems, we have developed pseudotyped viruses (HA-Bac) that present HA on the envelope of baculovirus. HA-Bac is water soluble for easy isolation, and HA can retain its native trimeric conformation on HA-Bac for better antibody stimulation. We vaccinated mice separately with either HA-Bac or purified HA and then collected mouse sera at several time-points for Western blotting and hemagglutinin inhibition assays. Our results show that both HA-Bac and purified HA elicited HA-inhibiting antibodies, but far less HA is needed for HA-Bac compared to purified HA to induce mouse immune responses. We also showed that HA-Bac are capable of agglutinating red blood cells, which serves as a convenient and safe tool to assay HA-neutralizing antibodies. We further expressed HA on the surface of cells and performed cell-based immunofluorescence assays with selected monoclonal antibodies and compared them with data generated from a traditional ELISA method. The results suggest that HA-Bac could provide a safe and convenient platform for antibody production and screening.
- Track 2: Influenza Vaccines and Vaccinnation
